Who Bcs Classification

Multisource (generic) products must satisfy the same standards as those applied to originator products. The manufacturer of a multisource (generic product) must demonstrate that its product: • satisfies the same standards as those applicable to the innovator product • provide assurance that it is clinically interchangeable with, i.e. Therapeutically equivalent or bioequivalent to, the innovator product. The manufacturer may therefore need to carry out a bioequivalence study: the data generated should provide a bridge between the (innovator) product for which safety and efficacy data are available and the generic products for which such data are not available. The WHO Technical Report Series (TRS) contain a number of annexes that manufacturers can consult regarding registration requirements for establishing the interchangeability of a multisource product with its comparator product, which is not normally the innovator product. These requirements must be met by any multisource product that is submitted for prequalification.
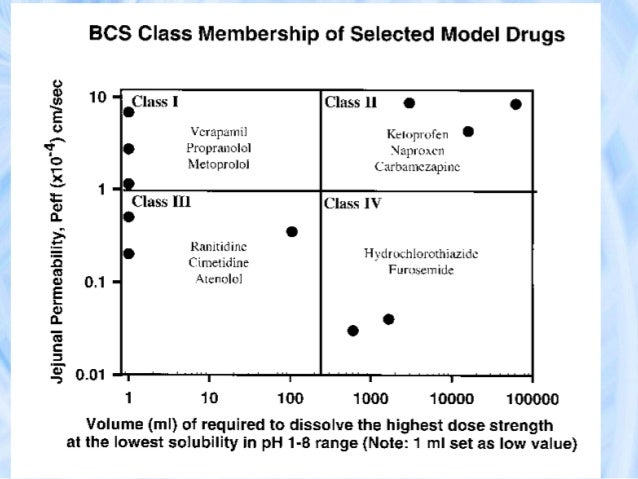
Detailed Brief on the Biopharmaceutical Classification System (BCS Classification), Dosage Form Trends and the approach. Fixed Dose Combinations Containing BCS Class 1, or Class 3, or Class 1 and 3. 4 See The Biopharmaceutics Classification System (BCS) Guidance at.
English Grammar Exercise - Beginner's Elementary English. The 10 questions English Grammar Exercise for beginners is only for elementary level students. Find out how good you are at basic English grammar with this multiple choice quiz. There is a gap in each sentence. Exercises for elementary and beginner students of English. Practise tenses, vocabulary and grammar in these interactive tests. Go back and try an exercise whenever you want – they’re free! Listening exercises for basic english beginners.
Bcs Classification System
In some cases, it may be possible to request that the requirement to conduct an in vivo study to establish bioequivalence be waived. The topic of biowaivers is discussed below. Design of bioequivalence studies The WHO Prequalification Team: medicines (PQTm) supports applicants in addressing specific scientific issues related to product development and design of bioequivalence studies that are intended to support an application for prequalification. It strongly recommends that applicants submit the final draft of their bioequivalence study protocol for review before embarking on the study. Questions on bioequivalence studies or final draft protocols can be sent to the. Comparator products for bioequivalence studies The quality, safety and efficacy of the innovator product, which was first authorized for marketing, were fully assessed and documented in pre-marketing studies and post-marketing monitoring schemes. The innovator product is therefore the most logical comparator product to use for establishing interchangeability.
SEO specialists are using this tool to check their hired freelancers as they need 100% unique content from them. Publishing the duplicated data is a big crime and in order to minimize this risk, almost each and every website uses this tool before publishing the content. Best plagiarism checker free online.
Fsx downloads free full version. Feb 11, 2014 Link is dead, please use the magnet If thepiratebay is blocked in your country us. Flight simulator full version free download - Mountain Flight Simulator 3D Full, Flight Simulator X demo, YS Flight Simulator, and many more programs.
Who Bcs Classification
Indeed, a generic pharmaceutical product should not be used as a comparator if an innovator product is available for this purpose. The risk with using a generic pharmaceutical product as a comparator is that similarity of future multisource products becomes progressively less reliable leading to a lack of interchangeability with the innovator. WHO has compiled lists of comparator products that are recommended for use in bioequivalence studies that aim to generate data for demonstrating bioequivalence of products invited for evaluation.